Background:
Bleeding and thrombosis remain the primary complications related to the use of extracorporeal membrane oxygenation (ECMO). To date, no single test or parameter has been identified to accurately predict the risk of these hemostatic complications. Thrombin generation may be the key marker for both thrombosis and bleeding, and may be influenced by inflammation. The aims of this study were to evaluate if novel laboratory tests including thrombin generation assay, neutrophil extracellular traps (NETs), microparticles (MPs), and red blood cell (RBC) membrane fragility/adhesion could better predict patients at risk for bleeding and/or thrombosis compared to routine laboratory tests. We hypothesized that ECMO related thrombosis is associated with increased thrombin generation and ECMO related bleeding is associated with decreased thrombin generation.
Methods:
An IRB approved prospective pilot study of patients requiring ECMO was conducted at Children's Hospital of Michigan. Patients were enrolled within 24 hours of ECMO cannulation after informed consent. Patients aged 0-17 years placed on venoarterial (VA) or venovenous (VV) ECMO were included. Patient demographics, baseline laboratory, clinical data, and novel assays were collected. Presented is the preliminary data of the first 16 patients enrolled. SPSS was used for data analysis.
Results:
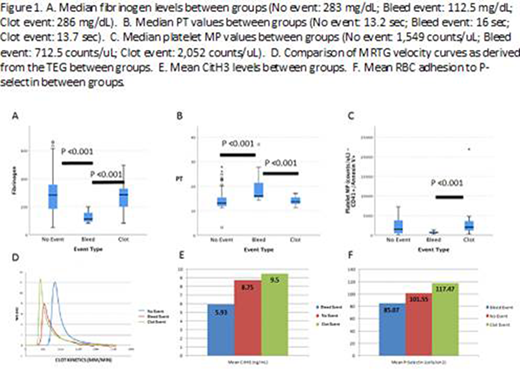
Of the 16 patients, there were 9 males and 7 females with the majority being cannulated for primary respiratory etiologies. Median age was 8.5 months with 6 patients being neonates (38%). Twelve patients (75%) survived the ECMO run, and 11 patients (69%) survived to hospital discharge. Laboratory data was grouped based on "no event" (N=94), "bleeding event" (N=13) and "clotting event" (N=21) days. There were no differences in the number of RBC, fresh frozen plasma, or cryoprecipitate transfusions between events. Platelet transfusions were used at higher volumes in bleeding events, p=0.011. Platelet counts did not differ between groups, whereas fibrinogen levels were significantly lower on days of bleeding events, p≤0.001 (Figure 1A). There were no differences seen in the activated partial thromboplastin time (aPTT) or activated clotting time (ACT) which are routinely used for monitoring anticoagulation. However, the prothrombin time (PT) was significantly prolonged in bleeding events with a median of 16 seconds (p=0.001), which may be related to the lower fibrinogen levels (Figure 1B). Thrombin generation was assessed using the maximum rate of thrombin generation (MRTG) value from the thromboelastograph (TEG) generated velocity curve (Figure 1D). Bleeding events had a significantly lower median MRTG of 7.93 mm/min compared to 12.36 mm/min in the no event group and 12.14 mm/min in the clotting event group (p=0.004). The thrombin generation assay results are in process. NETs were analyzed using an ELISA to detect citrullinated histone-3 (CitH3) levels (ng/mL). There were no significant differences seen in our population, however there is a trend toward increased levels in thrombotic events (Figure 1E). Both absolute MPs and platelet MPs were significantly increased in clotting events compared to bleeding events, p=0.003 and p≤0.001 respectively (Figure 1C). In our population, there was no evidence that hemolysis played a role in thrombosis as assessed by flow based RBC mechanical fragility testing. Of note, flow based RBC adhesion showed a non-significant trend toward increased adhesion to P-selectin. This finding requires further investigation because P-selectin is a primary site for adhesion on endothelial cells and plays a crucial role in coagulation (Figure 1F).
Conclusions:
Bleeding events on ECMO are associated with increased mortality. Our findings suggest that lower fibrinogen levels, prolonged PT and decreased thrombin generation (as assessed by the TEG) are associated with an increased risk for major bleeding. Increased levels of NETs, MPs and RBC adhesion have a trend towards increased thrombotic events. Thus, although preliminary, our results suggest that novel laboratory tests were effective in identifying bleeding events, and the trends seen in thrombotic events may allow for the use of new medications to reduce clotting in ECMO. The results illustrate the challenges in monitoring ECMO anticoagulation and additional study of novel tests are needed to identify and avoid clinical complications.
Tarasev:Functional Fluidics:Current Employment, Current equity holder in private company.Hines:Functional Fluidics:Current equity holder in private company.Chitlur:Biovertiv:Honoraria;Pfizer:Honoraria;Novo Nordisk:Consultancy, Honoraria;Takeda:Honoraria;Agios Pharmaceuticals:Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal